
Automation Solution – UNITYdx
Essential Automation Solution Components Technology
- Digital Monitoring of Safety Communications with site
- Tracking of Delivery Receipts and Acknowledgement Compliance
- Custom Configurations for Site Reminders and Email templates
- Complete Logical Separation of Sponsor (Customer) data
- Secure login using Multi-Factor Authentication
- Pre-Validated as per GAMP-5 guidelines
- Permission-based Access Control for Software Features, Read-only access
- 21CFR Part11 compliant Audit Trail Maintenance
Solution Drivers
- High Volume Communication that requires Tracking
- Highly Available Infrastructure – Secure, with Backup and Redundant Servers
- Secure Communication, Complete Audit Trail and Tracking
- Configurable, Regulatory Compliant
Service
- Simplified sync with Safety Systems via E2B R3 XML upload, EDI connection
- Automated assessment of Regulatory, Site and EC/IRB reporting timelines via Reporting Rules
- Detailed Compliance Reports with case-level and site-level as well as aggregated data presentation
- Custom Templates for Site Notifications and Reminders
- Cross-Reporting for multi-country trials
- Retrospective Reporting for New Sites joining a study
- Trial, Contact, Site and Product Masters for rapid integration with Safety and Clinical Databases
Benefits of Automation in Safety Document Distribution
Safety Document* Distribution Solution for Clinical Sites (* 7/15-day SUSARs,
DSURs, USRs)
- Safety Document Distribution with Digital Tracking:
- ENHANCED COMPLIANCE TRACKING
User-friendly dashboard with study/ site/ product-specific filters for data and reports
- ENHANCED COMPLIANCE TRACKING
- Secure Transmission of Reports
- SECURE TRANSMISSION
21 CFR Part11 compliant, Sign-in via secure access code received on verified email
- SECURE TRANSMISSION
- Reduce Human Effort and Probability of Error
- GREATER PROCESS EFFICIENCY
Automatic tracking and reminders, Cross-reporting, Retrospective reporting. Can be integrated with a CTMS for real-time updates
- GREATER PROCESS EFFICIENCY
About UNITYTMdx
Soterius offers its in-house tool SUSAR Notification, UNITYTMdx, which automates the sending and tracking of SUSARs to the Clinical Trial Sites. System integration and standardization allow UNITYTMdx to work with any standard safety database. Multi-channel communication hub ensures prompt, compliant, efficient, and secure communication between Clinical Trial sites, Safety Teams (CROs and Sponsors), and Clinical teams.
Challenges in Manual process of SUSAR Notifications
- Lack of regulatory compliant audit trails
- Excel-based tracking prone to data integrity issues
- Super busy Sites and Investigators do not respond
- Maintenance of contact information on Excel prone to error
- Multiple emails for1 SUSAR in case of multiple studies at a Site.
- Institutional policies block email delivery notifications
Implementation Strategies
Transition, Integrations and Training
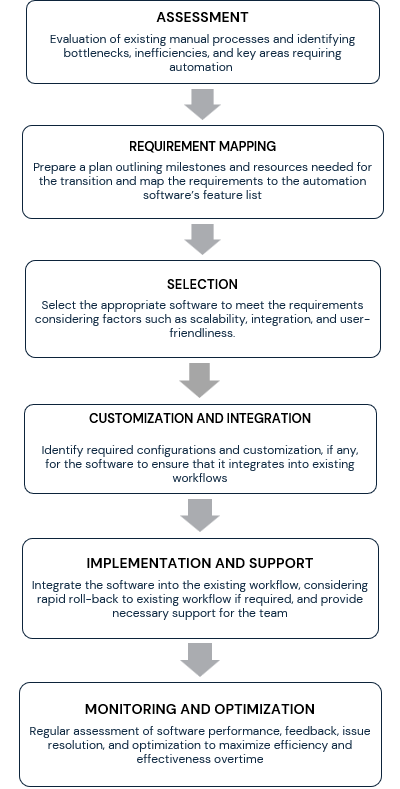
Key Takeaways
- Regulatory Compliance: Prompt SUSAR communication; Inspection Findings
- Manual Process of SUSAR Reporting is prone to errors, inadequate documentation and lack of audit trails
- Automation enhances process efficiency, mitigates risks in ensuring compliance and saves time and effort
- Ensuring data security with robust measures to protect sensitive information related to SUSAR notifications
- Successful Implementation needs a partner with Safety, Validation and Quality Assurance Expertise
- Aim is to have a Robust, Efficient and Compliant Solution for SUSAR / Safety Document Communication
About Soterius
Soterius is a strong team of pharma professionals who design customized, innovative, and cost-efficient processes for clinical safety, pharmacovigilance, and medical affairs. Our deep industry knowledge and up to date insights let us combine agile, people powered intelligence in pioneering customer centric solutions. Our innovative technology solutions include engagement tools and communications platforms to create a unified and compliant medical access facility. With a strong global presence, we provide comprehensive clinical and post marketed safety services, that include aggregate report writing, signal detection and management, global literature surveillance, risk management, case processing and regulatory reporting. We use state-of-the-art technologies to solve complex safety operations problems, be it case processing, intake, site reporting for clinical trials, or literature search and management. We have one of the most accurate solutions for case intake and case processing using AI.
We support companies from the initial development stage of a drug/vaccine to the approval and ultimate marketing of the therapy, supporting ongoing operations and regulatory commitments globally.
